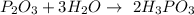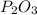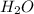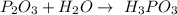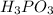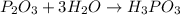Chemistry, 19.08.2019 05:30 alishajade
What is the chemical equation for diphosphorus trioxide + water > phosphorous acid?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1


Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
What is the chemical equation for diphosphorus trioxide + water > phosphorous acid?...
Questions

Chemistry, 19.06.2020 06:57


History, 19.06.2020 06:57

World Languages, 19.06.2020 06:57




Mathematics, 19.06.2020 06:57

History, 19.06.2020 06:57

Mathematics, 19.06.2020 06:57


Mathematics, 19.06.2020 06:57

Physics, 19.06.2020 06:57




Mathematics, 19.06.2020 06:57

Mathematics, 19.06.2020 06:57

Mathematics, 19.06.2020 06:57

History, 19.06.2020 06:57