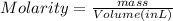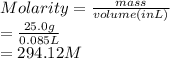Chemistry, 28.05.2021 14:50 brownw2005
What is the molarity when 25.0 g of the compound NaClO3 is placed in 85.0 mL of solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 04:31
Which of the following is an example of how telecommunication devices people do their jobs? a.) a security guard checks the time using a digital watch. b.) a banker does some quick math using a solar calculator. c.) a nurse uses a digital thermometer to take a patient’s temperature. d.) a construction worker reports in to his office using a cell phone.
Answers: 1

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2
You know the right answer?
What is the molarity when 25.0 g of the compound NaClO3 is placed in 85.0 mL of solution?...
Questions


Chemistry, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57


History, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57


Biology, 22.06.2020 23:57


Chemistry, 23.06.2020 00:57

History, 23.06.2020 00:57

French, 23.06.2020 00:57



Mathematics, 23.06.2020 00:57

Mathematics, 23.06.2020 00:57


History, 23.06.2020 00:57

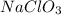 is placed in 85.0 mL of solution is 294.12 M.
is placed in 85.0 mL of solution is 294.12 M.