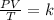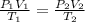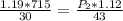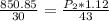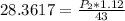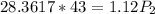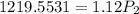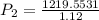Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
calculate the final pressure of a gas that is expanded from 725cm³ at 30C and 1.19 atm to 1.12cm³ at...
Questions




History, 12.04.2021 21:20


History, 12.04.2021 21:20

Mathematics, 12.04.2021 21:20

History, 12.04.2021 21:20






Mathematics, 12.04.2021 21:20

Mathematics, 12.04.2021 21:20

Chemistry, 12.04.2021 21:20



English, 12.04.2021 21:20

Mathematics, 12.04.2021 21:20