Chemistry, 02.08.2019 02:30 imstupid77
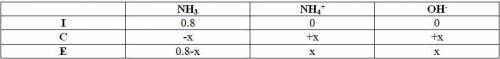
Ammonia, nh3, is a weak base with a kb = 1.8 x 10-5. in a 0.8m solution of ammonia, which has a higher concentration: ammonia (nh3) or its conjugate acid the ammonium ion (nh4+)?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2


Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Ammonia, nh3, is a weak base with a kb = 1.8 x 10-5. in a 0.8m solution of ammonia, which has a high...
Questions

Biology, 26.07.2019 07:00

Mathematics, 26.07.2019 07:00


Mathematics, 26.07.2019 07:00


Biology, 26.07.2019 07:00

Social Studies, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Biology, 26.07.2019 07:00

Mathematics, 26.07.2019 07:00

History, 26.07.2019 07:00

Biology, 26.07.2019 07:00

English, 26.07.2019 07:00

Advanced Placement (AP), 26.07.2019 07:00

Social Studies, 26.07.2019 07:00




Physics, 26.07.2019 07:00