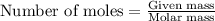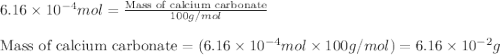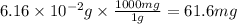Chemistry, 23.07.2019 10:00 coralstoner6793
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s) + 2h+(aq) > ca(2+)(aq) + co2(g) + h2o (l) 15.5ml of co2 was produced at 25*c and 738.0 mmhg how man moles of co2 were produced? how many milligrams of caco3 were consumed?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
To save time, you can approximate the initial mass of the solid to the nearest ±1 g. for example, if you are asked to add 14.3 g of copper, add between 13 g and 15 g. which of the following sets include two samples with an equal density? which all that apply below 15.4 g gold and 18.7 g silver 15.2 g copper and 50.0 g copper 20.2 g silver and 20.2 g copper 11.2 g gold and 14.9 g gold
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3


Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2
You know the right answer?
Acid precipitation dripping on limestone produces carbon dioxide by the following reaction: caco3(s...
Questions

Social Studies, 22.04.2021 09:50


Health, 22.04.2021 09:50



English, 22.04.2021 14:00

Business, 22.04.2021 14:00




English, 22.04.2021 14:00

Mathematics, 22.04.2021 14:00

Physics, 22.04.2021 14:00




Mathematics, 22.04.2021 14:00

History, 22.04.2021 14:00


Mathematics, 22.04.2021 14:00

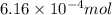 and the mass of calcium carbonate is 61.6 mg
and the mass of calcium carbonate is 61.6 mg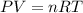
![25^oC=[25+273]K=298K](/tpl/images/0123/0054/df1f6.png)
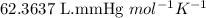

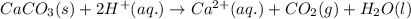
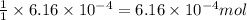 of calcium carbonate
of calcium carbonate