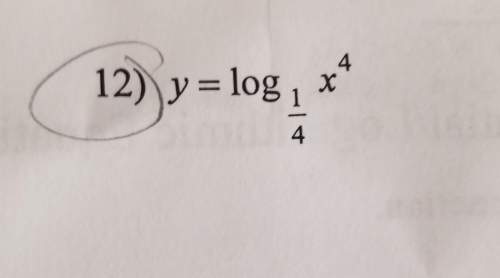Mathematics, 13.04.2020 20:13 petergriffin6772
How many liters of 4.0 M NaOH solution will react with 1.2 mol H2SO4? (Remember to balance the equation.)
H2SO4 + NaOH → Na2SO4 + H2O
1.6 L
1.2 L
0.90 L
0.60 L

Answers: 2


Another question on Mathematics

Mathematics, 21.06.2019 12:30
Hunter designs two flags for his adventure club. what is the length of the base, x, of the larger flag? enter your answer, as a decimal, in the box.
Answers: 2

Mathematics, 21.06.2019 13:40
Vip at (-2,7) dropped her pass and moved to the right on a slope of -9 where can you catch up to her to return her vip pass
Answers: 1

Mathematics, 21.06.2019 21:00
Reagan lives five miles farther from school than vanessa lives. write an expression to describe how far reagan lives from school
Answers: 1

Mathematics, 21.06.2019 22:00
Determine the domain and range of the given function. the domain is all real numbers all real numbers greater than or equal to –2{x: x = –2, –1, 0, 1, 2}{y: y = –2, –1, 0, 1, 2}. the range is all real numbers all real numbers greater than or equal to –2{x: x = –2, –1, 0, 1, 2}{y: y = –2, –1, 0, 1, 2}.
Answers: 1
You know the right answer?
How many liters of 4.0 M NaOH solution will react with 1.2 mol H2SO4? (Remember to balance the equat...
Questions

History, 05.03.2021 01:00



Chemistry, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00


English, 05.03.2021 01:00


History, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

History, 05.03.2021 01:00


Health, 05.03.2021 01:00



Mathematics, 05.03.2021 01:00

Biology, 05.03.2021 01:00