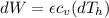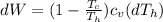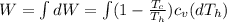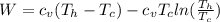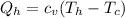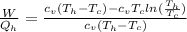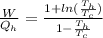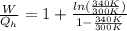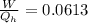Say that you are in a large room at temperature tc = 300 k. someone gives you a pot of hot soup at a temperature of th = 340 k. you set the bowl up so that as it cools to room temperature the heat first flows through a carnot engine. the soup has cv= (33 j/k). assume that the volume of the soup does not change. what fraction of the total heat qh that is lost by the soup can be turned into useable work by the engine? work / qh =

Answers: 3


Another question on Physics

Physics, 22.06.2019 04:10
Calculate the work done by an external agent during an isothermal compression of 1.00 mol of oxygen from a volume of 22.4 l at 10∘c and 1.0 atm pressure to 16.8l
Answers: 2

Physics, 22.06.2019 07:30
Choose all the answers that apply. our solar system: is in a spiral galaxy no longer includes pluto is made mostly of empty space is in the andromeda galaxy is the only known system that supports life
Answers: 3

Physics, 22.06.2019 11:30
Why is the energy that results from a roller coaster's position at the top of a hill referred to as potential energy?
Answers: 1

Physics, 23.06.2019 08:30
The momentum of a man riding his bicycle downhill can be calculated. the bicycle and the man have a combined mass of 40 kg. the velocity of the bicycle is 10 m/s. calculate the momentum.
Answers: 1
You know the right answer?
Say that you are in a large room at temperature tc = 300 k. someone gives you a pot of hot soup at a...
Questions

Social Studies, 12.12.2020 15:50

Geography, 12.12.2020 15:50

Geography, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


Physics, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50


Mathematics, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



Social Studies, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50




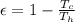
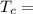 Temperature at the room
Temperature at the room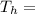 Temperature of the soup
Temperature of the soup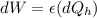
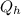 represents the input heat and at the same time is defined as
represents the input heat and at the same time is defined as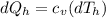
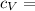 Specific Heat
Specific Heat