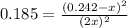Advanced Placement (AP), 26.02.2021 16:40 jdvazquez18p7a7vs
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.185. If 1.45 moles each of N 2(g)and O 2(g)are introduced in a container that has a volume of 6.00 liters and allowed to reach equilibrium at 300 K, what are the concentrations of N 2(g ) , O 2(g) ,and NO (g)at equilibrium?

Answers: 1


Another question on Advanced Placement (AP)

Advanced Placement (AP), 22.06.2019 09:30
In the workplace, it is important to listen to others’ opinions, communicate effectively, and lend a hand when needed. these are . to be a good team member, it is important to show . this means telling the truth, showing empathy, and making the right choices
Answers: 2


Advanced Placement (AP), 25.06.2019 00:30
Look back at act iv scene v to read over ophelia's interactions with claudius and gertrude as well as laertes. then, compare her dialogue to hamlet's dialogue and soliloquies previously analyzed in acts i–iv. what is ophelia's state of mind versus hamlet's?
Answers: 1

Advanced Placement (AP), 25.06.2019 01:00
What is tge speed of sound in dry air at 0°c
Answers: 1
You know the right answer?
For the equilibrium: 2 NO (g) ⇌ N 2(g)+ O 2(g)at 300 K, the equilibrium constant, Kc, is 0.18...
Questions




Mathematics, 29.06.2019 17:00



History, 29.06.2019 17:00



English, 29.06.2019 17:00

Social Studies, 29.06.2019 17:00

History, 29.06.2019 17:00



Social Studies, 29.06.2019 17:00

Mathematics, 29.06.2019 17:00


Mathematics, 29.06.2019 17:00

Chemistry, 29.06.2019 17:00

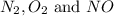 at equilibrium are 0.112 M, 0.112 M and 0.260 M
at equilibrium are 0.112 M, 0.112 M and 0.260 M = 1.45 mole
= 1.45 mole = 1.45 mole
= 1.45 mole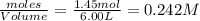
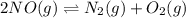
![K_c=\frac{[N_2]\times [O_2]}{[NO]^2}](/tpl/images/1150/8878/68b6f.png)