
Advanced Placement (AP), 04.03.2021 05:50 merrickrittany
For the following reaction, K > 1. Classify each of the reactants and products based on their strength as Bronsted-Lowry acids or bases. (C2H5)3N + HC9H7O4 —> C9H7O4- + (C2H5)3NH+
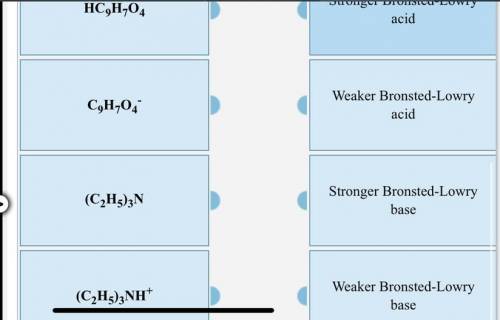

Answers: 1


Another question on Advanced Placement (AP)

Advanced Placement (AP), 24.06.2019 04:00
Brian has been working for a few years now and has saved a substantial amount of money. he now wants to invest 50 percent of his savings in a bank account where it will be locked for three years and gain interest. which type of bank account should brian open?
Answers: 2

Advanced Placement (AP), 24.06.2019 20:30
Which presentation option is also known as a screen of a presentation?
Answers: 1

Advanced Placement (AP), 24.06.2019 23:30
What are the major trends and specialties in psychology?
Answers: 1

Advanced Placement (AP), 25.06.2019 13:00
Which allows college students to work part time jobs to pay for educational expenses
Answers: 1
You know the right answer?
For the following reaction, K > 1. Classify each of the reactants and products based on their str...
Questions

Advanced Placement (AP), 03.03.2021 17:20

Biology, 03.03.2021 17:20


Mathematics, 03.03.2021 17:20

Mathematics, 03.03.2021 17:20


Health, 03.03.2021 17:20

Mathematics, 03.03.2021 17:20




History, 03.03.2021 17:20




Mathematics, 03.03.2021 17:20



Mathematics, 03.03.2021 17:20



