
Advanced Placement (AP), 10.03.2021 03:50 lnc0500
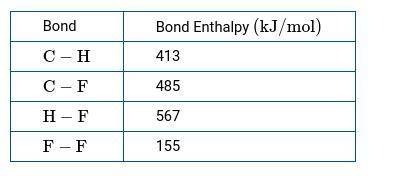
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g). b.) Use the bond enthalpies in the table below to calculate a numerical estimate of ΔH for the reaction.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 23.06.2019 14:40
Do you want free points and brainliest? answer this correctly and i got you : ) when scanning the road, glance away from the road ahead for a. at least one second b. no more than one second c. at least two seconds d. no more than two seconds
Answers: 2


Advanced Placement (AP), 24.06.2019 06:20
Which of the following is not one of the seven points from the pre-start checklist recommended by the nsc
Answers: 1

Advanced Placement (AP), 24.06.2019 07:00
With the use/abuse of alcohol, the heart is more efficient in the use of nutrients by the heart tissue.
Answers: 1
You know the right answer?
CH4(g) gas reacts with F2(g) to produce CH3F(g) and HF(g).
b.) Use the bond enthalpies in the table...
Questions

Mathematics, 25.05.2020 07:58

Mathematics, 25.05.2020 07:58

Computers and Technology, 25.05.2020 07:58

English, 25.05.2020 07:58

English, 25.05.2020 07:58

Mathematics, 25.05.2020 07:58

Mathematics, 25.05.2020 07:58

English, 25.05.2020 07:58


Social Studies, 25.05.2020 07:58



Advanced Placement (AP), 25.05.2020 07:58


Mathematics, 25.05.2020 07:58


History, 25.05.2020 07:58


Mathematics, 25.05.2020 07:58

English, 25.05.2020 07:58



