
Advanced Placement (AP), 08.04.2021 21:50 manlycool7543
Question 1:
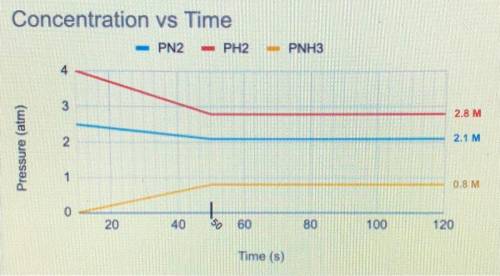
The balanced equation below is a reversible reaction. The concentration of each chemical species was
measured at various time points.
N2(g)+3 H2 (g) <—> 2NH3 (g)
a) When was equilibrium reached?
b) Write the equilibrium expression.
c) Would you expect the Keg to be greater than or less than 1? Justify your answer using data from the graph.

Answers: 2


Another question on Advanced Placement (AP)

Advanced Placement (AP), 22.06.2019 03:30
What does collaboration mean? ? and what is your favorite food? ?
Answers: 2

Advanced Placement (AP), 24.06.2019 00:20
Savings are transferred to businesses for investment through a) banks and financial institutions b) the federal government c) major corporations d) consumer purchases e) state and local governments
Answers: 1

Advanced Placement (AP), 25.06.2019 08:00
Which of the following would be paid for with a state’s capital budget? a) lawmakers’ salaries b) a new bridge c) the salaries of state troopers d) education
Answers: 1

Advanced Placement (AP), 25.06.2019 21:30
Most signs are diamond-shaped, with a yellow background and black letters or symbols. a. regulatory b. guide c. warning d. construction
Answers: 2
You know the right answer?
Question 1:
The balanced equation below is a reversible reaction. The concentration of each chemica...
Questions



Mathematics, 11.10.2020 09:01



Mathematics, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01





Mathematics, 11.10.2020 09:01

Social Studies, 11.10.2020 09:01

Mathematics, 11.10.2020 09:01



English, 11.10.2020 09:01

World Languages, 11.10.2020 09:01





