
Biology, 15.08.2019 07:30 SethSimunek
If an atom has one valence electron—that is, a single electron in its outer energy level—it will most likely form
a. one polar, covalent bond.
b. two nonpolar, covalent bonds.
c. two covalent bonds.
d. an ionic bond.
asap plz

Answers: 2


Another question on Biology


Biology, 22.06.2019 00:40
Which would best keep the oxygen cycle stable? a. cutting down jungles to create farmland b. eliminating parks in large cities c. dumping toxic chemicals into the ocean d. reducing the amount of deforestation d. reducing the amount of deforestation
Answers: 1

Biology, 22.06.2019 00:50
Which of the following is a macronutrient? carbohydrates lipids amino acids all of the above
Answers: 2

Biology, 22.06.2019 01:30
Select the correct answer. which term do biologists use to describe the average number of individuals of a species per unit area? a. carrying capacity b. population density c. minimal viable population d. survivability curve
Answers: 1
You know the right answer?
If an atom has one valence electron—that is, a single electron in its outer energy level—it will mos...
Questions

Mathematics, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

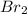 sharing of one electron takes place between both the bromine atoms.
sharing of one electron takes place between both the bromine atoms.

