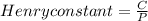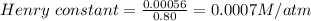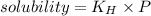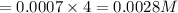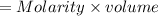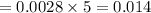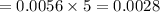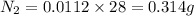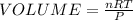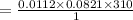The concentration of n2 in blood at 37 °c (body temperature) and atmospheric pressure (partial pressure of n2 = 0.80 atm) is 0.00056 mol/l. a deep-sea diver breathes compressed air with the partial pressure of n2 equal to 4.0 atm. assume that the total volume of blood in the body is 5.0 l. calculate the mass and volume (at 37°c and atmospheric pressure (p(n2) = 0.80 atm) of n2 gas released as a diver surfaces.

Answers: 2


Another question on Biology

Biology, 22.06.2019 03:00
What best describes the structure of the declaration of independence?
Answers: 2


Biology, 22.06.2019 05:50
Is there any species that went extinct in recent years due to natural causes (not caused by human interaction). if so, what caused it?
Answers: 3

Biology, 22.06.2019 07:40
What's the waste product of electrons and pyruvate combining in fermentation?
Answers: 2
You know the right answer?
The concentration of n2 in blood at 37 °c (body temperature) and atmospheric pressure (partial press...
Questions



Social Studies, 23.07.2021 04:30



Mathematics, 23.07.2021 04:30

Computers and Technology, 23.07.2021 04:30

Chemistry, 23.07.2021 04:30

History, 23.07.2021 04:30

Mathematics, 23.07.2021 04:30


Mathematics, 23.07.2021 04:30

Mathematics, 23.07.2021 04:30


Computers and Technology, 23.07.2021 04:30

Computers and Technology, 23.07.2021 04:30

English, 23.07.2021 04:30