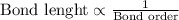Biology, 14.11.2019 19:31 curlyloo01
N₂ has a shorter inter-nuclear distance than o₂ because 1. nitrogen is less electro-negative than oxygen. 2. false; o₂ has a shorter bond length because oxygen is more electro-negative than nitrogen. 3. false; o₂ has a shorter bond length because oxygen has six valence electrons but nitrogen has only five. 4. n₂ is triple-bonded but o₂ is double-bonded. 5. n₂ is more polar than o₂. 6. false; n₂ and o₂ have the same bond lengths because both are covalent diatomic molecules.

Answers: 2


Another question on Biology



Biology, 22.06.2019 02:20
Astudent analyzed ears of corn that demonstrated two traits in the f2 kernels, purple or white colors and smooth on wrinkled shapes. a tabulation of 135 individual kernels gave the following results: purple and smooth = 75 white and smooth = 28 purple and wrinkled = 24 white and wrinkled = 8 what would be the only phenotype present in the f1 ger
Answers: 3

Biology, 22.06.2019 03:30
In pea plants, the allele for inflated pod seed, i, is dominant over the allele for constricted pod seed, i. the punnett square shows a cross for this trait. which offspring will be homozygous dominant
Answers: 2
You know the right answer?
N₂ has a shorter inter-nuclear distance than o₂ because 1. nitrogen is less electro-negative than ox...
Questions



Mathematics, 02.09.2019 03:50

Mathematics, 02.09.2019 03:50

Mathematics, 02.09.2019 03:50


Social Studies, 02.09.2019 03:50

Social Studies, 02.09.2019 03:50

Geography, 02.09.2019 03:50


Mathematics, 02.09.2019 03:50

History, 02.09.2019 03:50

Geography, 02.09.2019 03:50


Biology, 02.09.2019 03:50


Mathematics, 02.09.2019 03:50


Mathematics, 02.09.2019 03:50

Computers and Technology, 02.09.2019 03:50