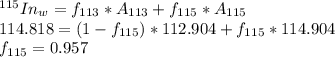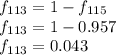Biology, 21.02.2020 23:35 joelpimentel
G Indium has two naturally occurring isotopes: 113In with an atomic weight of 112.904 amu, and 115In with an atomic weight of 114.904 amu. If the average atomic weight for In is 114.818 amu, calculate the fraction-of-occurrences of 115In isotope. Round off the answer to three significant figures.

Answers: 3


Another question on Biology

Biology, 21.06.2019 23:00
Use the drop down menus to match each example to the fossil topic discussed then to show how the fossil record gives evidence of evolution
Answers: 1

Biology, 21.06.2019 23:10
Afamily has a y-linked disease that affects the father. what is the chance of a male offspring inheriting the same disease? oa. 100% ob. 50% oc. 25% d. 0%
Answers: 1

Biology, 22.06.2019 03:00
The unique structure of the neuron is dedicated to the efficient and rapid transmission of neural signals. the relationship between neurons, the spinal cord, and the brain constitutes an elaborate communication system throughout the human body. all but one of the functions listed below are a result of this interaction.
Answers: 1

Biology, 22.06.2019 11:20
2polnis which of the following is an advantage of an in vitro experiment?me
Answers: 1
You know the right answer?
G Indium has two naturally occurring isotopes: 113In with an atomic weight of 112.904 amu, and 115In...
Questions



English, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30

Spanish, 25.09.2019 21:30

Social Studies, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30

History, 25.09.2019 21:30

Chemistry, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30


English, 25.09.2019 21:30




Mathematics, 25.09.2019 21:30

English, 25.09.2019 21:30

Mathematics, 25.09.2019 21:30


Mathematics, 25.09.2019 21:30

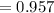
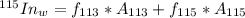 ------(1)
------(1)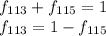 ---------(2)
---------(2)