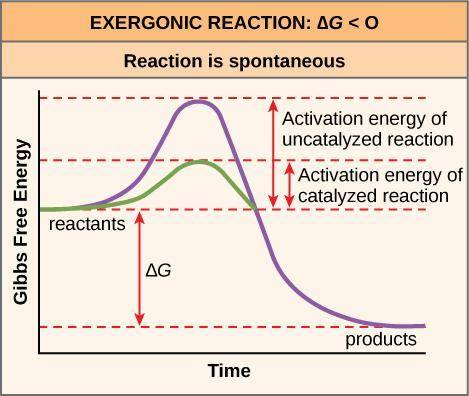
Many exergonic reactions fail to happen at a reasonable rate (e. g. conversion of diamonds to charcoal). This is due to the fact that their activation energy may be too high to overcome. Which of the following correctly describes the reason for this
O The free energy of the transition state is much lower than the free energy of the reactants
O The free energy of the transition state is much higher than the free energy of the reactants.
Many exergonic reactions fail to happen at a reasonable rate (e. g. conversion of diamonds to charcoal). This is due to the The enzyme that catalyzes the reaction needs time to interact chemically with the substrate(s).
The interaction between the enzyme and substrate(s) involve a decrease in entropy, which can't happen input of energy

Answers: 2


Another question on Biology

Biology, 22.06.2019 02:30
Did you know that a single bee would have to go to over 2 million flowers to make a single pound of honey?
Answers: 1

Biology, 22.06.2019 04:30
Which of the following describes a boom period? a. as one population increases, another population decreases. b. as one population increases, the other population also increases. c. as one population decreases, another population increases. d. as one population decreases, another population also decreases
Answers: 2

Biology, 22.06.2019 08:30
Which macromolecule catalyzes chemical reactions this be considered enzyme chemical reactions thus he considering enzymes
Answers: 1

You know the right answer?
Many exergonic reactions fail to happen at a reasonable rate (e. g. conversion of diamonds to charco...
Questions





Spanish, 23.10.2021 19:10

Health, 23.10.2021 19:10



Mathematics, 23.10.2021 19:10


Mathematics, 23.10.2021 19:10



Mathematics, 23.10.2021 19:10

Social Studies, 23.10.2021 19:10