
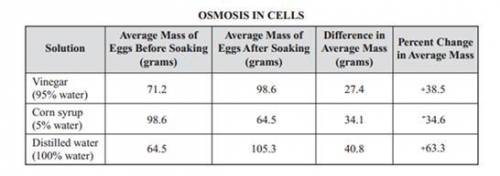
8. A classic example of osmosis in the classroom involves placing an egg in water or syrup. The egg first sits in vinegar until the shell is dissolved. Then, it is placed in a glass of either tap water or syrup. The mass of the egg is measured. The results are in the chart below. Use what you know about osmosis to explain why the egg increased in mass in the distilled water but decreased in mass in the corn syrup.

Answers: 2


Another question on Biology

Biology, 22.06.2019 02:40
Lucia is walking barefoot in her yard. she accidentally steps on a nail. how will her nervous system work to generate a reaction? arrange the eventschronologically.
Answers: 1


Biology, 22.06.2019 08:30
Describe how a non-resistant staphylococcus aureus bacterium can produce a bacterium that is resistant to methicillin
Answers: 1

Biology, 22.06.2019 12:00
Define the apical impulse and describe its normal location, size, and duration. which abnormal conditions may affect the location of the apical impulse? explain the mechanism producing normal first and second heart sounds. describe the effect of respiration on the heart sounds. describe the characteristics of the first heart sound and its intensity at the apex of the heart and at the base. describe the characteristics of the second heart sound and its intensity at the apex of the heart and at the base.
Answers: 1
You know the right answer?
8. A classic example of osmosis in the classroom involves placing an egg in water or syrup. The egg...
Questions



Computers and Technology, 12.04.2020 01:51

Mathematics, 12.04.2020 01:51

Business, 12.04.2020 01:51

Spanish, 12.04.2020 01:51

Mathematics, 12.04.2020 01:51


Mathematics, 12.04.2020 01:51

Mathematics, 12.04.2020 01:51




Mathematics, 12.04.2020 01:51


Biology, 12.04.2020 01:51




Mathematics, 12.04.2020 01:51



