
Biology, 23.10.2020 23:20 donavery24
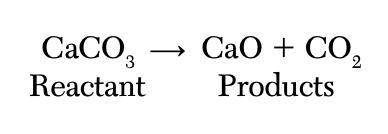
When calcium carbonate (CaCO3) is heated, it decomposes to form calcium oxide (CaO) and carbon dioxide (CO2). The equation below shows this reaction. In this reaction, the mass of CaCO3
A. is less than the mass of CaO plus the mass of CO2
B. is greater than the mass of CaO plus the mass of CO2
C. equals the mass of CaO plus the mass of CO2
D. equals the mass of CaO minus the mass of CO2

Answers: 3


Another question on Biology

Biology, 22.06.2019 02:40
Which must be kept in mind when determining if an explanation is correct? check all that apply.which must be kept in mind when determining if an explanation is correct? check all that apply.
Answers: 2

Biology, 22.06.2019 07:30
In this assignment, you will analyze an example of speciation by researching the finches of the galapagos islands. then you will answer questions to construct explanations and draw conclusions based on the information you gather. someone plz !
Answers: 3


Biology, 22.06.2019 13:30
Methane gas created by a cows flatulence especially in a large herd is a greenhouse gas. true or false.
Answers: 2
You know the right answer?
When calcium carbonate (CaCO3) is heated, it decomposes to form calcium oxide (CaO) and carbon dioxi...
Questions




Mathematics, 11.01.2021 21:40

Mathematics, 11.01.2021 21:40

Mathematics, 11.01.2021 21:40

Mathematics, 11.01.2021 21:40

Business, 11.01.2021 21:40





English, 11.01.2021 21:40



Mathematics, 11.01.2021 21:40






