Biology, 10.12.2020 18:50 marcucciisabella
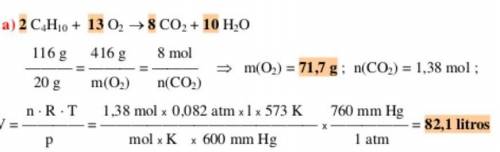
1. Determina la masa de los reactivos y productos de la siguiente ecuación química 2C4H10 +
1302 → 8CO2 + 10H20 demostrando la ley de proporciones definidas. Datos: Carbono (C)
12g, Hidrogeno (H) 1g, Oxigeno (0)165 m

Answers: 2


Another question on Biology

Biology, 22.06.2019 10:30
How do the evolutionary chart and the video support the claim that all living things are made up of cells?
Answers: 3

Biology, 22.06.2019 13:40
1. -define adaptation2 explain darwin's theories of descent with modification and natural selection in detail3. -explain how each of these provides evidence for evolution: a. -fossil record, including superposition and transitional fossilsb-anatomy, including homologous structuresc-biological molecules, including dna and proteins4. -explain the difference between convergent and divergent evolution.5. -define and give three examples of artificial selection6. -define and give one example of coevolution.7. -explain biodiversity and how it benefits humans.8. -explain a type of population lest affected by environmental change.
Answers: 3


Biology, 22.06.2019 19:30
Which two molecules generated by the krebs cycle pass their high-energy electrons to the electron transport chain? a. nadh b. c6h12o6 c. fadh2 d. nad+
Answers: 1
You know the right answer?
1. Determina la masa de los reactivos y productos de la siguiente ecuación química 2C4H10 +
1302 →...
Questions


Social Studies, 06.10.2020 03:01





Chemistry, 06.10.2020 03:01

Mathematics, 06.10.2020 03:01


Social Studies, 06.10.2020 03:01




English, 06.10.2020 03:01


Chemistry, 06.10.2020 03:01

Mathematics, 06.10.2020 03:01



Mathematics, 06.10.2020 03:01