HELP ME/Science
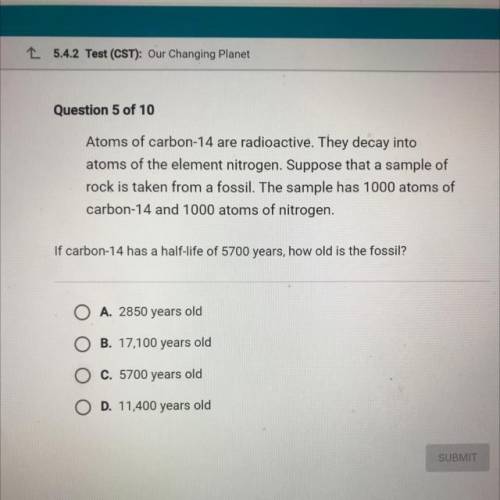
Atoms of carbon-14 are radioactive. They decay into
atoms of the element nitr...

Biology, 10.12.2020 20:30 amortegaa805
HELP ME/Science
Atoms of carbon-14 are radioactive. They decay into
atoms of the element nitrogen. Suppose that a sample of
rock is taken from a fossil. The sample has 1000 atoms of
carbon-14 and 1000 atoms of nitrogen.
If carbon-14 has a half-life of 5700 years, how old is the fossil?
O A. 2850 years old
O B. 17,100 years old
O c. 5700 years old
O D. 11,400 years old
IDRIT

Answers: 3


Another question on Biology

Biology, 22.06.2019 04:10
What noticeable trend from this graph might be used to make a conclusion?
Answers: 1

Biology, 22.06.2019 05:20
The mammal pictured below is a silvery mole rat. which statement is an inference based on the picture? the animal is ugly. the animal has hairless feet with sharp claws. the animal has prominent upper and lower incisor teeth. the animal likely has poor vision since its eyes are so small.
Answers: 2

Biology, 22.06.2019 16:40
Astudent sees several ants walking up a wall following the exact same trail that an ant took earlier. she wants to apply the scientific method to determine how the ants detected the trail. which of these steps would come first in her application of the scientific method? perform an experiment by cleaning the scent away from part of the trail. draw a conclusion that the ants follow a scent trail. make a prediction about what the ants will do after she cleans away part of the trail. hypothesize that the ants are following a scent trail that the first ant left.
Answers: 1

Biology, 22.06.2019 18:00
Does all the energy stored by the phytoplankton reach the top level of the pyramid
Answers: 1
You know the right answer?
Questions


Mathematics, 29.12.2020 07:00





Social Studies, 29.12.2020 07:00

Mathematics, 29.12.2020 07:00


Mathematics, 29.12.2020 07:00

Arts, 29.12.2020 07:00

Computers and Technology, 29.12.2020 07:00

Arts, 29.12.2020 07:00

Mathematics, 29.12.2020 07:00



Spanish, 29.12.2020 07:10

Mathematics, 29.12.2020 07:10




