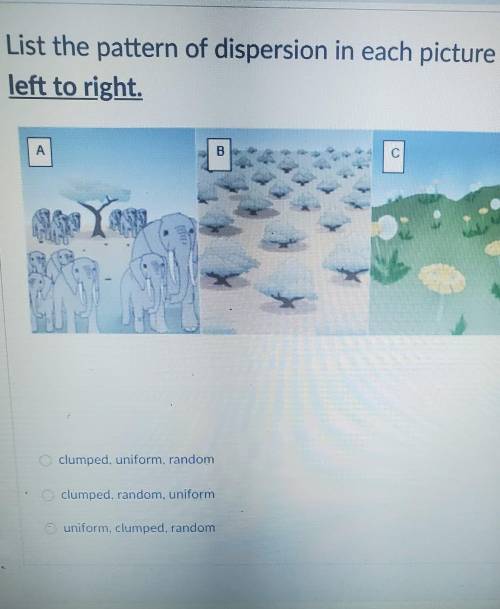
Help I will mark brainliest
...

Answers: 3


Another question on Biology

Biology, 21.06.2019 16:00
Every compound can be represented by a chemical formula. a chemical formula is the set of symbols showing the elements that make up a compound and their proportions. water’s chemical formula is h 2 2 o. that means a molecule of water contains two atoms of hydrogen and one atom of oxygen chemically bonded. a molecule is a group of two or more atoms bonded together. molecules can range in size from two to thousands of atoms. a molecule of a compound is the smallest unit of that compound. every molecule of water has all the physical and chemical properties of water. that’s because every molecule of water is made of the same elements in the same proportions: two hydrogen atoms and one oxygen! which of the following is a true statement? a every molecule of the same compound will have different properties. b every molecule of a compound has the same proportion of elements. c molecules can be physically separated into smaller units of a compound. d molecules cannot be represented by chemical formulae.
Answers: 1

Biology, 21.06.2019 22:00
How does the molecular clock work? a. it analyzes the brain functionality of two different species.b. it examines and compares the physical characteristics of two different species.c. it illustrates relationships between two different species.d. it compares the number of mutations that exist in the dna of two different species.
Answers: 1


Biology, 22.06.2019 16:30
Energy stored in the bonds that hold together the atoms and molecules of all substances is called a. kinetic energy. b. electrical energy. c. mechanical energy. d. chemical energy.
Answers: 1
You know the right answer?
Questions



English, 15.11.2019 08:31


Spanish, 15.11.2019 08:31





Biology, 15.11.2019 08:31

Social Studies, 15.11.2019 08:31

Mathematics, 15.11.2019 08:31

History, 15.11.2019 08:31


Computers and Technology, 15.11.2019 08:31




Mathematics, 15.11.2019 08:31

Computers and Technology, 15.11.2019 08:31