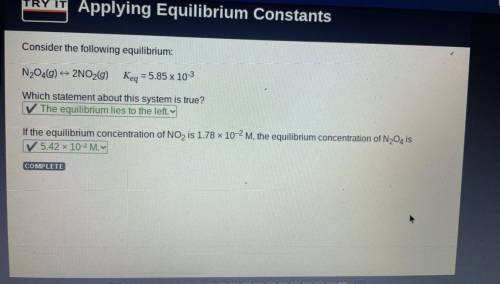
Consider the following equilibrium:
N204(0) 2NO2(g)
Keq = 5.85 x 10-3
Which statement...

Biology, 20.04.2021 18:40 eweqwee3147
Consider the following equilibrium:
N204(0) 2NO2(g)
Keq = 5.85 x 10-3
Which statement about this system is true?
-✓ The equilibrium lies to the left.
If the equilibrium concentration of NO2 is 1.78 x 10-2 M, the equilibrium concentration of N204 is
-5.42 x 10-2M.

Answers: 2


Another question on Biology

Biology, 22.06.2019 04:30
Sexual reproduction in the parent cell will result in offspring with a) identical genetic information. b) half the genetic information. c) double the genetic information. d) four times the genetic information.
Answers: 1

Biology, 22.06.2019 06:00
Mineral rich water heated by newly found oceanic crust escapes through cracks in the ocean floor called
Answers: 2

Biology, 22.06.2019 08:00
When a doggo starts to pace around in the same line for a hand full of days. what would that mean?
Answers: 2

Biology, 22.06.2019 12:00
Can a trait be both polygenic and have multiple alleles? explain why or why not.
Answers: 2
You know the right answer?
Questions

Mathematics, 18.09.2020 01:01

Social Studies, 18.09.2020 01:01

Biology, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Social Studies, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

English, 18.09.2020 01:01

Geography, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01

Mathematics, 18.09.2020 01:01



