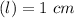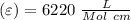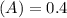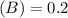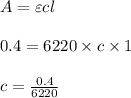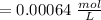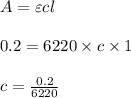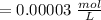Using and spectrophotometer, you measure 2 dilutions of NADH, and get absorbance values of 0.4 for sample A, and 0.2 for sample B. You know that the path length is 1 cm, and the extinction coefficient for NADH is 6220 (L Morcm). Using the Lambert-Beer Law equation (below), calculate the concentrations of sample A Select] and Sample B (Select ] A = log10 () = Ecl Where: A- Absorbance C- Concentration (mol 1 - Path length (cm) E = molar decadic extinction coefficient L mol. cm 1o - Intensity of the incident light 1 - Intensity of the transmitted Night

Answers: 3


Another question on Biology


Biology, 21.06.2019 18:00
What do science, technology, and disease have to do with each other?
Answers: 1

Biology, 21.06.2019 19:30
Give the biological term for: structures that display characteristics of living organisms only within living cells
Answers: 1

You know the right answer?
Using and spectrophotometer, you measure 2 dilutions of NADH, and get absorbance values of 0.4 for s...
Questions





History, 12.02.2021 21:10

Biology, 12.02.2021 21:10

Mathematics, 12.02.2021 21:10


English, 12.02.2021 21:10





Advanced Placement (AP), 12.02.2021 21:10

Mathematics, 12.02.2021 21:10


Chemistry, 12.02.2021 21:10


Biology, 12.02.2021 21:10

Mathematics, 12.02.2021 21:10

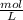 and concentration of sample B is 0.00003
and concentration of sample B is 0.00003