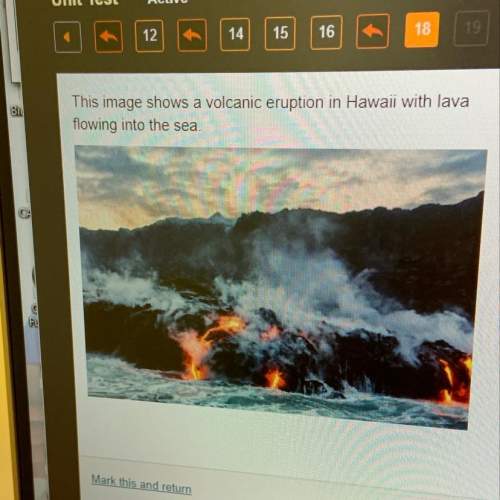
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statement about the atoms in ammonia (NH3)?
a. Each hydrogen atom has a partial positive charge: the nitrogen atom has a partial negative charge.
b. Ammonia has an overall positive charge. Ammonia has an overall negative charge.
c. The nitrogen atom has a partial positive charge: each hydrogen atom has a partial negative charge.
d. There are covalent bonds between the hydrogen atoms and polar bonds between each hydrogen atom and the nitrogen atom

Answers: 3


Another question on Biology

Biology, 21.06.2019 13:30
Which is the best way to define a common in environmental science a. a worthless resource b. shared resource c. a rare resource d. a typical resource
Answers: 1

Biology, 21.06.2019 16:20
Which element is used in photosynthesis found in atmosphere and fixed by bacteria?
Answers: 2

Biology, 22.06.2019 02:10
Scenario #2 in 2001, a population of 2,500 poison dart frogs lived in the amazon rain forest. due to increased deforestation, the population dwindled to 25 frogs in 2019. new government regulations were enacted in 2022, successfully putting an end to the deforestation of the amazon rain forest. once deforestation was stopped, the poison dart frog population was able to recover. by 2050, the population reached 8,000 frogs, of that population, 20 are homozygous recessive for being spotted (ss genotype). q2- ? q- p- p2- 2pq-
Answers: 2

Biology, 22.06.2019 07:00
In 2001, records showed that local stocks of fish were down worldwide. yet, records of harvests indicated that fish were being taken at records rates. what was actually happening?
Answers: 3
You know the right answer?
Nitrogen (N) is more electronegative than hydrogen (H). Which of the following is a correct statemen...
Questions



Mathematics, 17.10.2021 06:10


Social Studies, 17.10.2021 06:10



History, 17.10.2021 06:10

English, 17.10.2021 06:10

Mathematics, 17.10.2021 06:10

Mathematics, 17.10.2021 06:10

History, 17.10.2021 06:10






Mathematics, 17.10.2021 06:10

Business, 17.10.2021 06:10

Mathematics, 17.10.2021 06:10