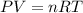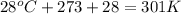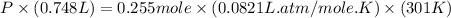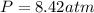Chemistry, 21.07.2019 04:00 robertstoll81
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of this gas. (r= 0.0821 l ∙ atm / mol ∙ k) 0.784 atm 8.42 atm 0.00842 atm 7.84 × 10-4 atm none of the above

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

You know the right answer?
Question 1 a sample of 0.255 mole of gas has a volume of 748 ml at 28°c. calculate the pressure of t...
Questions

History, 08.07.2019 02:30

English, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30


Mathematics, 08.07.2019 02:30



Mathematics, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30



Health, 08.07.2019 02:30


Health, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30


Mathematics, 08.07.2019 02:30

Mathematics, 08.07.2019 02:30


Physics, 08.07.2019 02:30