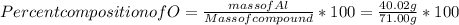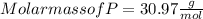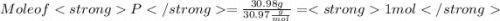Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 22:00
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2
You know the right answer?
When a 30.98-g sample of phosphorus reacts with oxygen, a 71.00-g sample of phosphorus oxide is form...
Questions




Mathematics, 18.03.2021 01:20

History, 18.03.2021 01:20



Physics, 18.03.2021 01:20

Chemistry, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Mathematics, 18.03.2021 01:20


Chemistry, 18.03.2021 01:20