
Chemistry, 20.07.2019 23:00 tashai3138
The acid dissociation constant for an acid dissolved in water is equal to the a. equilibrium constant b. equilibrium constant times the concentration of water c. equilibrium constant divided by the concentration of water d. equilibrium constant times the equilibrium constant of water

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Consider the balanced equation below. n2h4 + 2h2o2 n2 + 4h2o what are the mole ratios of hydrazine (n2h4) to hydrogen peroxide (h2o2) and hydrazine to water? 1: 2 and 1: 4 1: 3 and 1: 4 1: 2 and 3: 5 1: 3 and 3: 5
Answers: 3

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2
You know the right answer?
The acid dissociation constant for an acid dissolved in water is equal to the a. equilibrium consta...
Questions




Mathematics, 06.09.2021 04:30



Mathematics, 06.09.2021 04:30

History, 06.09.2021 04:30


English, 06.09.2021 04:30

History, 06.09.2021 04:30

English, 06.09.2021 04:30


English, 06.09.2021 04:40

Biology, 06.09.2021 04:40



English, 06.09.2021 04:40

Mathematics, 06.09.2021 04:40

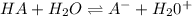
![K_{eq}=\frac{[A^-][H_3O^+]}{[HA][H_2O]}](/tpl/images/0113/3718/a0805.png)
![K_a=K_{eq}\times [H_2O]=\frac{[A^-][H_3O^+]}{[HA]}](/tpl/images/0113/3718/c2e7c.png)


