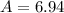Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of li-6 is 7.42% and the relative abundance of li-7 is 92.58%. based on this data alone, calculate the average atomic mass for lithium to the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 01:30
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
Lithium-6 has a mass of 6.0151 amu and lithium-7 has a mass of 7.0160 amu. the relative abundance of...
Questions





Mathematics, 04.02.2020 09:50



Mathematics, 04.02.2020 09:50

Spanish, 04.02.2020 09:50

History, 04.02.2020 09:50

Biology, 04.02.2020 09:50


Social Studies, 04.02.2020 09:50

History, 04.02.2020 09:51





Biology, 04.02.2020 09:51

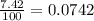
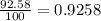
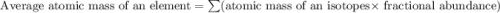
![A=\sum[(6.0151\times 0.0742)+(7.0160\times 0.9258)]](/tpl/images/0113/1342/3dcbf.png)