
Chemistry, 20.07.2019 21:30 leeahnnfoster
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf = –393.5 kj/mol), and h2 o(g) (mc016-3.jpghf = –241.82) in the reaction: mc016-4.jpg what is the enthalpy of combustion, per mole, of butane?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 18:00
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
You know the right answer?
Butane c4 h10 (-1.jpghf = –125.7), combusts in the presence of oxygen to form co2 (g) (mc016-2.jpghf...
Questions

Chemistry, 10.07.2019 09:00




Mathematics, 10.07.2019 09:00





Social Studies, 10.07.2019 09:00

History, 10.07.2019 09:00

History, 10.07.2019 09:00

Chemistry, 10.07.2019 09:00


Mathematics, 10.07.2019 09:00





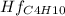 = -125.7 kJ/mol
= -125.7 kJ/mol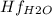 = -241.82 kJ/mol
= -241.82 kJ/mol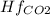 = -393.5 kJ/mol
= -393.5 kJ/mol

