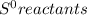Chemistry, 20.07.2019 13:30 FireStorm7327
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h12o6(s) + 6o2(g) —-> 6co2(g) + 6h2o(l) +131 j/k -131 j/k +262j/k -262 j/k +417j/k

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1


Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
What is the value for δs°reaction for the following reaction, given the standard entropy values c6h1...
Questions

Mathematics, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00


Mathematics, 17.07.2019 06:00

Biology, 17.07.2019 06:00





Social Studies, 17.07.2019 06:00





Biology, 17.07.2019 06:00

Chemistry, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00

History, 17.07.2019 06:00

Mathematics, 17.07.2019 06:00

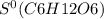 = 212.1 J/K.mol
= 212.1 J/K.mol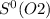 = 205.0 J/K.mol
= 205.0 J/K.mol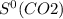 = 213.6 J/K.mol
= 213.6 J/K.mol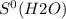 = 69.9 J/K.mol
= 69.9 J/K.mol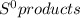 - ∑
- ∑