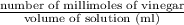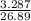Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Suppose that 26.89 ml of vinegar solution requires 33.23 ml of the 0.09892 m sodium hydroxide soluti...
Questions

Mathematics, 12.01.2021 18:50

History, 12.01.2021 18:50

Arts, 12.01.2021 18:50

Mathematics, 12.01.2021 18:50


Mathematics, 12.01.2021 18:50






Chemistry, 12.01.2021 18:50





Mathematics, 12.01.2021 18:50


Mathematics, 12.01.2021 18:50

Mathematics, 12.01.2021 18:50