
Chemistry, 20.07.2019 12:00 aROSSconpollo
Write a balanced half-reaction for the oxidation of manganese ion mn 2 to permanganate ion mno−4 in basic aqueous solution. be sure to add physical state symbols where appropriate.

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1
You know the right answer?
Write a balanced half-reaction for the oxidation of manganese ion mn 2 to permanganate ion mno−4 in...
Questions

Mathematics, 24.11.2020 01:00

Spanish, 24.11.2020 01:00


Computers and Technology, 24.11.2020 01:00

English, 24.11.2020 01:00



History, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Mathematics, 24.11.2020 01:00

Spanish, 24.11.2020 01:00


Social Studies, 24.11.2020 01:00


History, 24.11.2020 01:00




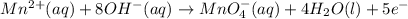
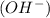 at that side where the less number of hydrogen are present.Now balance the charge.
at that side where the less number of hydrogen are present.Now balance the charge.

