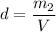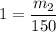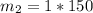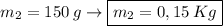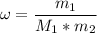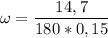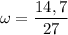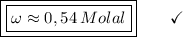Chemistry, 20.07.2019 12:00 Ruthsybel9754
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? assume the density of water is 1.00 g/ml?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
What is the molality of a solution made by dissolving 14.7 g of c6h12o6 into 150.0 ml of water? ass...
Questions

Mathematics, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30

History, 05.02.2021 20:30

Chemistry, 05.02.2021 20:30


Mathematics, 05.02.2021 20:30

Chemistry, 05.02.2021 20:30


Mathematics, 05.02.2021 20:30


Mathematics, 05.02.2021 20:30


Mathematics, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30



English, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30

History, 05.02.2021 20:30

Mathematics, 05.02.2021 20:30