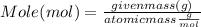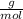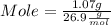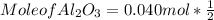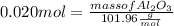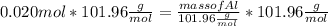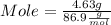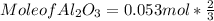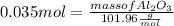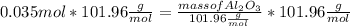Chemistry, 20.07.2019 07:30 awkwardkid0123
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g of al? 3mno2 (s) + 4al(s) -> 3mn (s) + 2al2o3 (s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 11:00
As air becomes more dense, (select all that apply) o. air weighs less o. gas molecules are closer together o. air is colder o. air weighs more o. gas molecules are further apart o. air is hotter
Answers: 3

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

You know the right answer?
What mass of aluminum oxide is produced from the reaction of 4.63 g of manganese dioxide and 1.07 g...
Questions

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50


Computers and Technology, 25.02.2021 21:50


Social Studies, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50

Mathematics, 25.02.2021 21:50


Mathematics, 25.02.2021 21:50


English, 25.02.2021 21:50



Mathematics, 25.02.2021 21:50