
Chemistry, 20.07.2019 04:30 MalikaJones
Consider a monatomic gas of particles each with mass m. what is vx, rms=⟨v2x⟩−−−−√, the root mean square (rms) of the x component of velocity of the gas particles if the gas is at an absolute temperature t? express your an

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2
You know the right answer?
Consider a monatomic gas of particles each with mass m. what is vx, rms=⟨v2x⟩−−−−√, the root mean sq...
Questions


English, 18.10.2019 17:50

Mathematics, 18.10.2019 17:50


Health, 18.10.2019 17:50

Mathematics, 18.10.2019 17:50

Mathematics, 18.10.2019 17:50

Business, 18.10.2019 17:50





Mathematics, 18.10.2019 17:50



Mathematics, 18.10.2019 18:00




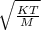 when n = 1 mole or it equals:
when n = 1 mole or it equals: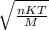 when n not 1 mole
when n not 1 mole

