

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 05:50
What are the coefficients to balance the following equation? ba+br=babr2
Answers: 1
You know the right answer?
Cl2(g) + 2nabr(aq) 2nacl(aq) + br2(l) the reaction given above is a single replacement reaction beca...
Questions


Mathematics, 24.05.2020 11:57


Mathematics, 24.05.2020 11:57

Mathematics, 24.05.2020 11:57

Mathematics, 24.05.2020 11:57

History, 24.05.2020 11:57

History, 24.05.2020 11:57

English, 24.05.2020 11:57


World Languages, 24.05.2020 11:57


Biology, 24.05.2020 11:57

Physics, 24.05.2020 11:57

Biology, 24.05.2020 11:57


Spanish, 24.05.2020 11:57

Mathematics, 24.05.2020 11:57

Mathematics, 24.05.2020 11:57

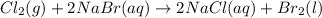
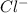 in
in 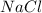 and
and 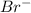 in
in 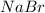 donates electrons to form
donates electrons to form 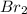 .
.


