
Chemistry, 19.07.2019 13:00 alejandra216
For the reaction: h2(g) + cl2(g) → 2hcl(g), how many moles hcl will be produced from 10.0 g of h2? the reaction occurs in the presence of excess cl2

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
For the following reaction, 5.65 grams of oxygen gas are mixed with excess hydrochloric acid . assume that the percent yield of water is 86.4 %. hydrochloric acid(aq) + oxygen(g) water(l) + chlorine(g) what is the ideal yield of water ? grams what is the actual yield of water ? grams
Answers: 1

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1
You know the right answer?
For the reaction: h2(g) + cl2(g) → 2hcl(g), how many moles hcl will be produced from 10.0 g of h2?...
Questions

Biology, 11.01.2022 01:00


Mathematics, 11.01.2022 01:00

Mathematics, 11.01.2022 01:00





Social Studies, 11.01.2022 01:00

Health, 11.01.2022 01:00








Computers and Technology, 11.01.2022 01:00


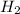 = 10 g
= 10 g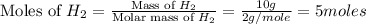
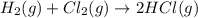
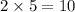 moles of HCl
moles of HCl


