Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

You know the right answer?
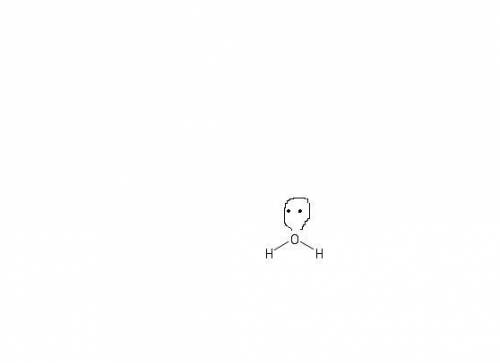
How does a lone pair contribute to molecular shape? a. it is too small to affect the molecule's sha...
Questions




Mathematics, 23.04.2021 05:20

Mathematics, 23.04.2021 05:20






Law, 23.04.2021 05:20



Mathematics, 23.04.2021 05:20


Mathematics, 23.04.2021 05:20

Geography, 23.04.2021 05:20


Mathematics, 23.04.2021 05:20

Mathematics, 23.04.2021 05:20