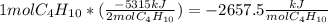Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2 , mc031-2.jpghf = –393.5 kj/mol ) and water (h2 o, mc031-3.jpghf = –241.82 kj/mol) according to the equation below. mc031-4.jpg what is the enthalpy of combustion (per mole) of c4h10 (g)? use mc031-5.jpg. –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1


Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
Butane (c4 h10(g), mc031-1.jpghf = –125.6 kj/mol) reacts with oxygen to produce carbon dioxide (co2...
Questions


Mathematics, 22.02.2021 21:40


Mathematics, 22.02.2021 21:40


Mathematics, 22.02.2021 21:40


Mathematics, 22.02.2021 21:40

Geography, 22.02.2021 21:40


Mathematics, 22.02.2021 21:40

Spanish, 22.02.2021 21:40





Chemistry, 22.02.2021 21:40

History, 22.02.2021 21:40


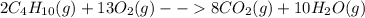
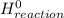 = Σ
= Σ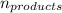 Δ
Δ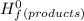 -Σ
-Σ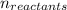 Δ
Δ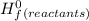
![[{8*(-393.5kJ/mol)}+{10*(-241.82kJ/mol)}]-[{2*(-125.6kJ/mol)}+13*(0 kJ/mol)}]](/tpl/images/0106/1493/0d4a2.png) =[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]
=[-3148kJ/mol+(-2418.2kJ/mol)]-[(-251.2kJ/mol)+0]