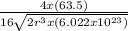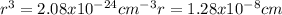Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 23.06.2019 02:00
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
Copper has a face-centered cubic unit cell. the density of copper is 8.96 g/cm3. calculate a value f...
Questions




Social Studies, 21.01.2020 08:31





English, 21.01.2020 08:31


English, 21.01.2020 08:31

English, 21.01.2020 08:31



Mathematics, 21.01.2020 08:31


Mathematics, 21.01.2020 08:31

Mathematics, 21.01.2020 08:31


Mathematics, 21.01.2020 08:31

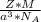
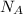 is Avogadro's number, and a is the edge length.
is Avogadro's number, and a is the edge length.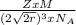
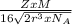
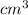 =
=