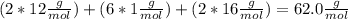Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
An organic compound is composed of 38.7% c, 9.70% h, 51.6% o. the compound has a molecular formula m...
Questions

History, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57



History, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Arts, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

Mathematics, 23.06.2020 10:57

History, 23.06.2020 10:57

Computers and Technology, 23.06.2020 10:57



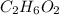
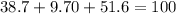

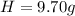
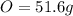
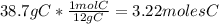
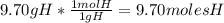
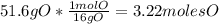
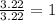

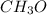 , but you should find the molecular formula taking in account the molar mass of the compound, so:
, but you should find the molecular formula taking in account the molar mass of the compound, so: