Chemistry, 18.07.2019 06:30 archiecom55
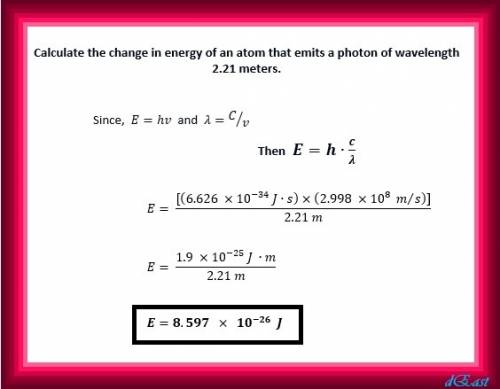
Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s constant is 6.626 x 10-34 joule seconds, the speed of light is 2.998 x 108 m/s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 02:00
What is the difference between a substance "getting wet" and "being dissolved" in a liquid at the particulate level?
Answers: 3
You know the right answer?
Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s c...
Questions

Chemistry, 22.07.2019 22:00


Geography, 22.07.2019 22:00

Social Studies, 22.07.2019 22:00



English, 22.07.2019 22:00


Mathematics, 22.07.2019 22:00


Mathematics, 22.07.2019 22:00

Social Studies, 22.07.2019 22:00

Physics, 22.07.2019 22:00

Mathematics, 22.07.2019 22:00



Chemistry, 22.07.2019 22:00



Biology, 22.07.2019 22:00