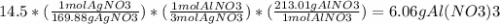Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

You know the right answer?
If 14.5 grams of silver nitrate (molar mass =169.88g/mol) reacts with aluminum chloride. how many gr...
Questions

Health, 29.06.2019 07:00

English, 29.06.2019 07:00

Mathematics, 29.06.2019 07:00

Chemistry, 29.06.2019 07:00

Spanish, 29.06.2019 07:00

Mathematics, 29.06.2019 07:00



History, 29.06.2019 07:00

Advanced Placement (AP), 29.06.2019 07:00



Mathematics, 29.06.2019 07:00

English, 29.06.2019 07:00


English, 29.06.2019 07:00

History, 29.06.2019 07:00


History, 29.06.2019 07:00

Biology, 29.06.2019 07:00