Chemistry, 04.12.2019 22:31 cabriantenpenny
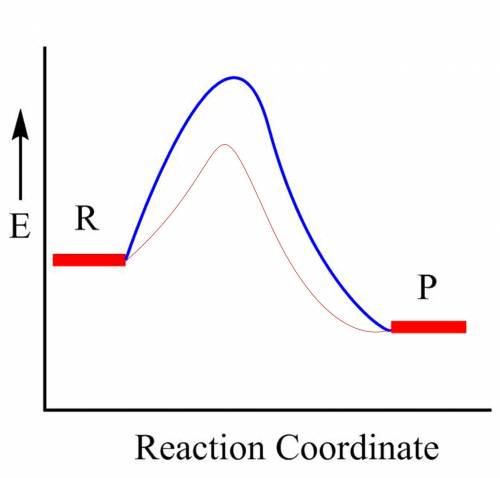
How would an energy diagram for a reaction that used a catalyst differ from an energy diagram without a catalyst?
the reactants will be at a higher potential energy.
the products will be at a higher potential energy.
the barrier for activation energy will be lower.
the barrier for activation energy will be higher.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
How would an energy diagram for a reaction that used a catalyst differ from an energy diagram withou...
Questions


History, 27.01.2020 21:31





English, 27.01.2020 21:31





Mathematics, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31

World Languages, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31

History, 27.01.2020 21:31


Mathematics, 27.01.2020 21:31