From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the ave...

Chemistry, 09.10.2019 19:30 estefaniapenalo
From the diagram, when the temperatures of reactants are raised from 20°c to 30°c:
a. the average kinetic energy doubles.
b. the number of molecules doubles.
c. the total number of collisions doubles.
d. the number of molecules having er or greater doubles.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Questions




English, 02.09.2020 01:01


History, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01

Biology, 02.09.2020 01:01


Mathematics, 02.09.2020 01:01



Mathematics, 02.09.2020 01:01

Mathematics, 02.09.2020 01:01

Biology, 02.09.2020 01:01




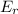 which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.
which means now double number of molecules will be able to cross the energy barrier and hence the formation of product increases and hence the rate increases to double.

