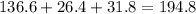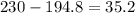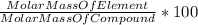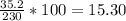Chemistry, 17.07.2019 07:30 sarahbennett11p4yxlb
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen. the rest is oxygen. what is the mass percent of oxygen in the compound? 11.48%

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 13:30
Which of these statements describes the size of an atom? a. an atom is larger than a sheet of aluminum foil. b. an atom is small but can be seen with just our eyes. c. an atom is the size of a plastic building block. d. an atom is tiny and cannot be seen without magnification.
Answers: 2
You know the right answer?
A230 g sample of a compound contains 136.6 g of carbon, 26.4 g of hydrogen, and 31.8 g of nitrogen....
Questions

History, 28.01.2020 04:31




Mathematics, 28.01.2020 04:31

Social Studies, 28.01.2020 04:31


Mathematics, 28.01.2020 04:31

Mathematics, 28.01.2020 04:31


Mathematics, 28.01.2020 04:31

Mathematics, 28.01.2020 04:31

History, 28.01.2020 04:31

Arts, 28.01.2020 04:31

Chemistry, 28.01.2020 04:31