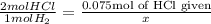Chemistry, 17.07.2019 06:30 lizzzzi7908
In the reaction mg (s) + 2hcl (aq) -> h2 (g) + mgcl (aq), how many moles of hydrogen gas will be produced from 75.0 milliliters of 1.0 m hcl in an access of mg?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2
You know the right answer?
In the reaction mg (s) + 2hcl (aq) -> h2 (g) + mgcl (aq), how many moles of hydrogen gas will be...
Questions

Mathematics, 25.06.2019 15:30

English, 25.06.2019 15:30



History, 25.06.2019 15:30

English, 25.06.2019 15:30

English, 25.06.2019 15:30


Mathematics, 25.06.2019 15:30


Health, 25.06.2019 15:30


Mathematics, 25.06.2019 15:30

Mathematics, 25.06.2019 15:30


Mathematics, 25.06.2019 15:30

Social Studies, 25.06.2019 15:30


Chemistry, 25.06.2019 15:30

Advanced Placement (AP), 25.06.2019 15:30