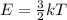Chemistry, 17.07.2019 04:30 thegreentnt5025
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid (hcl) and potassium iodide (ki). when the reaction is completed at 273k there are approximately 250,000 collisions per mole of reactant. you run the experiment again at 350k. which of the following would you expect to be the number of collisions recorded at 350k a. 2000 b. 700,000 c. 0 d. 200,000

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2


You know the right answer?
You are experimenting on the effect of temperature on the rate of reaction between hydrochloric acid...
Questions


Geography, 26.07.2019 01:30


Physics, 26.07.2019 01:30

Physics, 26.07.2019 01:30

Geography, 26.07.2019 01:30

Biology, 26.07.2019 01:30




History, 26.07.2019 01:30

History, 26.07.2019 01:30



History, 26.07.2019 01:30