

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
When iron rusts in air iron3 oxide is produced. how many moles of oxygen react with 28.0 mol of iron...
Questions


Mathematics, 07.10.2019 20:30

Social Studies, 07.10.2019 20:30

Mathematics, 07.10.2019 20:30

Engineering, 07.10.2019 20:30

Mathematics, 07.10.2019 20:30



Mathematics, 07.10.2019 20:30

Computers and Technology, 07.10.2019 20:30


English, 07.10.2019 20:30

History, 07.10.2019 20:30



Mathematics, 07.10.2019 20:30




 .
.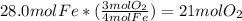
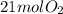 is produced.
is produced.

