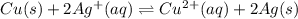Chemistry, 16.07.2019 16:00 emileehogan
Which represents the correct equilibrium constant expression for the reaction below? cu(s)+2ag+(aq)< -> cu2+(aq)+2ag(s)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 07:30
To separate a mixture of hard candies nd marbles the most efficient method would be
Answers: 3

You know the right answer?
Which represents the correct equilibrium constant expression for the reaction below? cu(s)+2ag+(aq)...
Questions





Mathematics, 06.06.2020 22:58







Social Studies, 06.06.2020 22:58

Chemistry, 06.06.2020 22:58

Computers and Technology, 06.06.2020 22:58






![k_{eq}=\frac{[Cu^{2+}]}{[Ag^+]^2}](/tpl/images/0096/8739/3c835.png)